Nanoscale imaging of clinical specimens using conventional and rapid-expansion pathology
Bucur, O., Fu, F., Calderon, M. et al. Nanoscale imaging of clinical specimens using conventional and rapid-expansion pathology. Nat Protoc 15, 1649–1672 (2020). https://doi.org/10.1038/s41596-020-0300-1
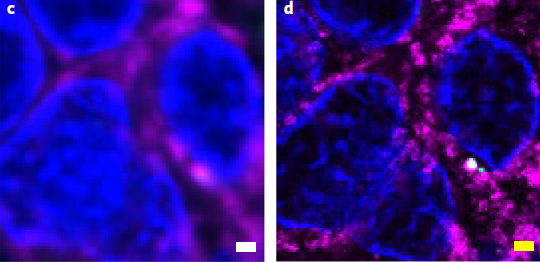
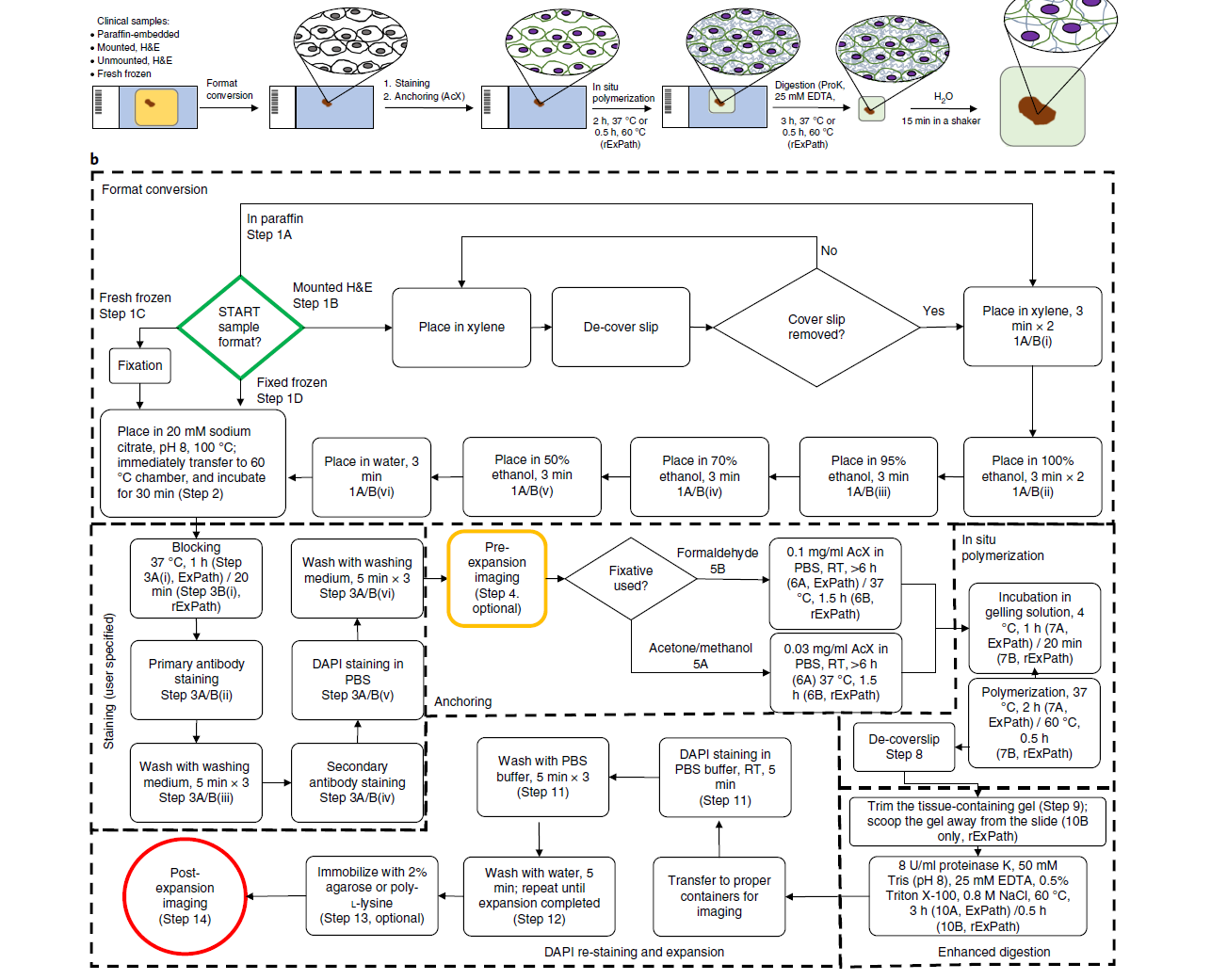
In pathology, microscopy is an important tool for the analysis of human tissues, both for the scientific study of disease states and for diagnosis. However, the microscopes commonly used in pathology are limited in resolution by diffraction. Recently, we discovered that it was possible, through a chemical process, to isotropically expand preserved cells and tissues by 4–5× in linear dimension. We call this process expansion microscopy (ExM). ExM enables nanoscale resolution imaging on conventional microscopes. Here we describe protocols for the simple and effective physical expansion of a variety of human tissues and clinical specimens, including paraffin-embedded, fresh frozen and chemically stained human tissues. These protocols require only inexpensive, commercially available reagents and hardware commonly found in a routine pathology laboratory. Our protocols are written for researchers and pathologists experienced in conventional fluorescence microscopy. The conventional protocol, expansion pathology, can be completed in ~1 d with immunostained tissue sections and 2 d with unstained specimens. We also include a new, fast variant, rapid expansion pathology, that can be performed on <5-µm-thick tissue sections, taking <4 h with immunostained tissue sections and <8 h with unstained specimens.